Intensive care treatment
What medications and therapies help with COVID-19?
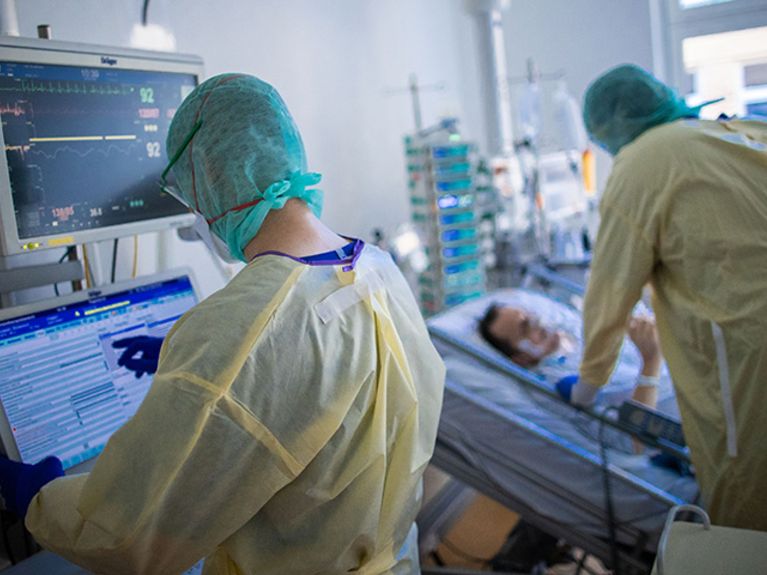
Five out of one hundred people infected with SARS-CoV-2 quickly become life-threatening and receive intensive medical therapy (Image: dpa/Jens Büttner).
We have been living with the Corona pandemic for a year now. A lot has changed in patient treatment since then. Senior physician Harald Prüß explains which drugs are effective and which are not - and how the survival chances of intensive care patients have developed.
It is now more than a year since the first COVID-19 patients arrived at German hospitals. A study published by a German research team in the journal "The Lancet Respiratory Medicine" shows that much has improved this year. The scientists analyzed health insurance billing data of discharged COVID-19 patients from all hospitals in Germany in 2020. One important finding: the proportion of patients who required intensive care treatment decreased significantly over the course of the year. According to the analyses, between March and May 2020, one in three hospital patients suffering from COVID-19 still required intensive care. In the fall of 2020, only half as many patients were still in need of intensive care. By the end of the year - i.e., during the period when the number of cases was rising sharply - intensive care physicians were treating only 14 out of every hundred patients who had to be hospitalized for COVID-19. Thus, physicians are increasingly successful in treating patients with relatively severe courses and thus preventing very severe courses that require intensive care.
The treatment of patients with severe COVID-19 is extremely complex. Overall, about twelve to 15 percent of all infected patients require hospital-based therapy. They develop an excessive reaction of the immune system, the coagulation system goes crazy. As a result, they come to the hospital with disorders of the respiratory tract, the skin, the cardiovascular system, kidney failure, dangerous blood clots or even a stroke.
Harald Prüß is research group leader at the German Center for Neurodegenerative Diseases (DZNE) and senior physician at the Department of Neurology with Experimental Neurology at Charité (Image: DZNE).
"Five out of one hundred infected patients quickly become life-threatening and receive intensive medical therapy," says scientist and senior physician Harald Prüß. At the German Center for Neurodegenerative Diseases (DZNE), he heads the research group "Autoimmune Encephalopathies" and is also a senior physician at the Department of Neurology with Experimental Neurology at Charité - Universitätsmedizin Berlin. He stresses the importance of preventing severe courses from worsening to the point of needing ventilation. "Invasively ventilated intensive care patients with COVID-19 unfortunately still do not have a good prognosis," Prüß knows. "The data show that one in two of them still dies, at the beginning of the pandemic and today." Those most affected are the elderly with risk factors for cardiovascular disease and/or pre-existing conditions.
Medications and therapies for COVID-19 treatment
The good news is that there are now numerous scientifically based, effective medications and treatment options for patients who do not require invasive ventilation, meaning they can still breathe on their own. "The absolute standard of therapy and now included in medical guidelines are steroids such as dexamethasone," says Prüß. The cortisone significantly attenuates the exaggerated immune reaction. "Studies show that severely ill patients with cortisone are hospitalized for a shorter period of time, are ventilated less often and die less frequently." However, the drug should be administered carefully.
Patients also receive preventive medications such as heparin, which controls the out-of-control clotting system. "By far the most important thing for patients, however, is oxygen; after all, in COVID-19, it is the lungs that are most affected," the senior physician adds. Oxygen can significantly reduce mortality.
"Under cortisone, severely ill patients are hospitalized for a shorter period of time, require less ventilation, and die less often."
Other agents, however, have proven to be flops. "Worldwide, more than 100 drugs have been tested for efficacy against COVID-19 in recent months," Prüß says. These include cardiovascular drugs, attenuating immunomodulators, antibiotics, vitamins or agents developed against other viruses such as hepatitis C, influenza, SARS or MERS. "So far, none of these substances has made it into widespread use," sums up the neurologist, who himself treats patients in intensive care units. "Even remdesevir is now no longer recommended by the WHO."
Remdesivir and serum therapy are only effective to a limited extent
The drug works against other viruses by suppressing, so to speak, the copying machine in human cells that the pathogens use to multiply rapidly. Remdesivir was last used - without great success - in Africa to treat patients in the Ebola epidemic. It has recently been approved in the European Union for early COVID-19 therapy when severely ill patients are on oxygen but not yet ventilated.
According to Prüß, so-called serum therapy has also been overestimated. This involves taking blood from people who have already been infected with Sars-CoV-2. The plasma, i.e. the liquid portion of the blood, contains antibodies against the virus. Serum therapy, which was already used during the Spanish flu, experienced a revival in China and the USA during the Corona pandemic - despite a lack of evidence of efficacy. Patients are also being treated this way at several clinics in this country. "However, the majority of studies show that there is no convincing effect," says Prüß.
Effective antibody therapy
"Far more promising are quality-tested, synthetically produced monoclonal antibodies," explains Harald Prüß. These are proteins active in the immune system that bind to the target molecule at only a single site. Therapy with monoclonal antibodies means that they are administered directly to the patient, whereby the antibody recognizes the virus, binds to it and neutralizes it. Two antibodies from U.S. manufacturers Regeneron (casirivimab/imdevimab) and Eli Lilly (bamlanivimab) are already approved in the United States. "All the studies that led to emergency approval there were able to show that the antibodies help in the early phase of COVID-19," Prüß said.
"Antibodies can help alleviate symptoms and prevent hospitalization in the early stages of COVID-19."
The Ministry of Health also recently purchased 200,000 doses of these U.S. antibodies for Germany. "The antibodies are already being used sporadically in this country at Charité and other university hospitals," says Prüß. "They should now also be used quickly in the outpatient setting to prevent worsening of symptoms and hospitalization as early as possible." Unlike active vaccination - in which the immune system itself forms antibodies to injected attenuated pathogens or pathogen components - the human body receives ready-made antibodies against the virus with this so-called passive vaccination.
Together with his team from the German Center for Neurodegenerative Diseases and the Charité, Prüß is working on passive vaccination himself. The scientists have just completed the planning for phase I and II studies. Currently, industrial partner Miltenyi Biotec is producing antibodies in large quantities. "As soon as we have the antibodies in hand, we can start the studies," says Prüß. "With a little luck, our antibodies will be ready in Germany next fall."
Readers comments